FUEL CELLS - THE SOURCE OF SUSTAINABLE ENERGY
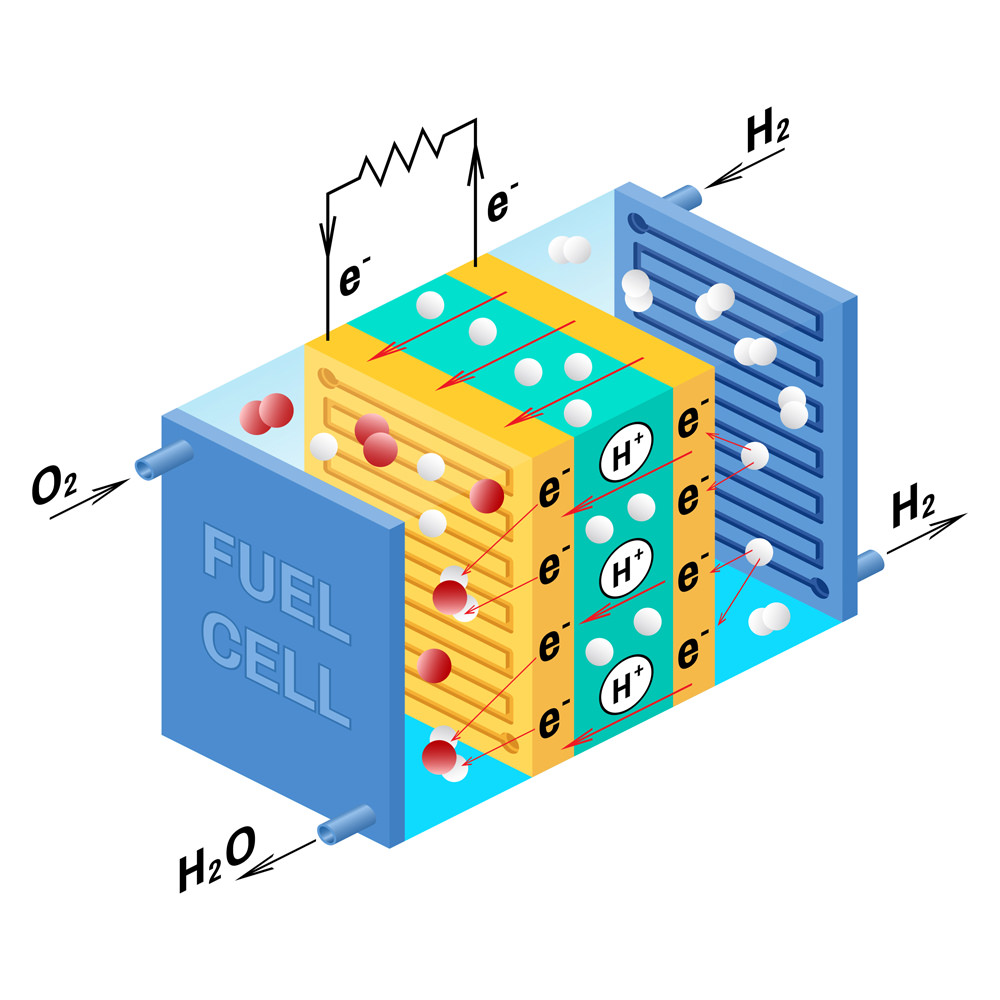
A fuel cell is an electrochemical energy generation device that combines hydrogen with oxygen in the air to produce electricity, with water and heat as the only by-products.
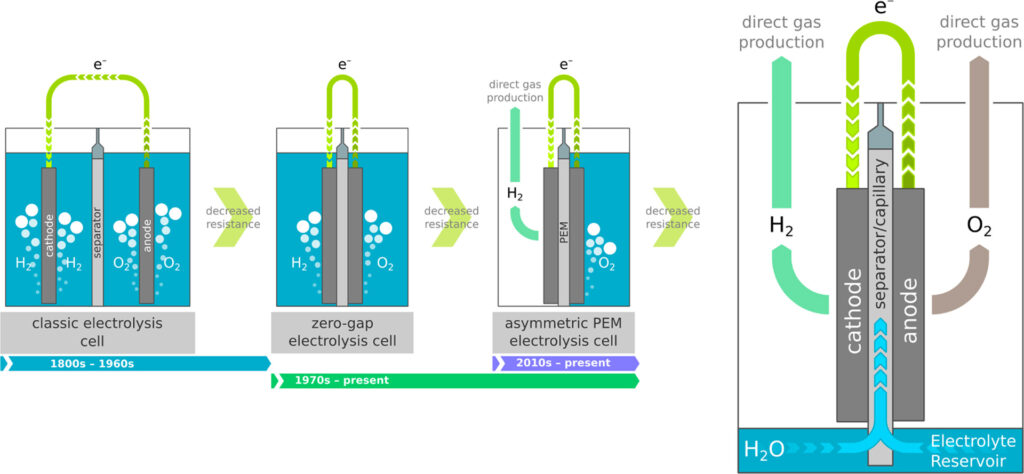
Safe, functional and efficient fuel cell systems, toghether with their supporting infrastructure of electrolyzers and hydrogen refuelling stations (HRS), will play a fundamental role in the transition to sustainable energy.
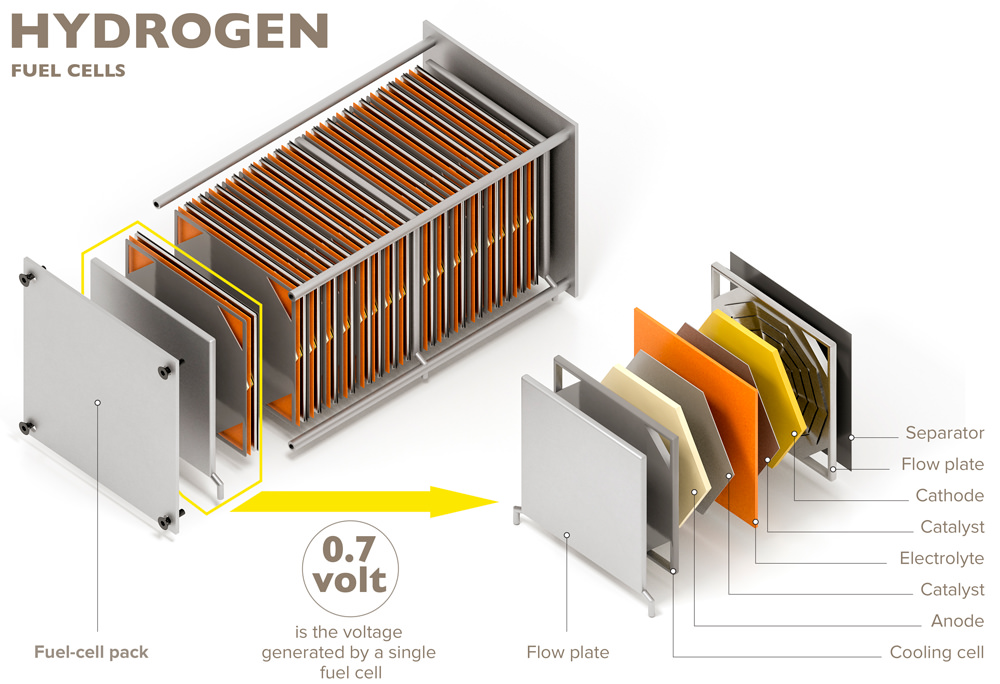
The scope of today’s fuel cell systems extends from small scale portable applications in the Watt range to large field installations with power supply in the MW scale.